| 英文缩写 | 英文全称 | 中文全称 |
| | Clinical Trial | 临床试验 |
| IND | Investigational New Drug Application | 新药临床研究申请 |
| NDA | New Drug Application | 新药申请 |
| | Multi-center Trial | 多中心试验 |
| NMPA | National Medical Products Administration | 国家药品监督管理局 |
| ICH | International Conference on Harmonization | 国际协调会议 |
| IRB | Institutional Review Board | 机构审查委员会 |
| FDA | Food and Drug Administration | 美国食品与药品管理局 |
| GCP | Good Clinical Practice | 药物临床试验质量管理规范 |
| GMP | Good Manufacturing Practice | 药品生产质量管理规范 |
| HGRAC | China Human Genetic Resources Management Office | 中国人类遗传资源管理办公室(人遗办) |
| IEC | Independent Ethics Committee | 独立伦理委员会 |
| CSA | Clinical Study Application | 临床研究申请 |
| CTA | Clinical Trial Application | 临床试验申请 |
| | Sponsor | 申办者 |
| CRO | Contract Research Organization | 合同研究组织 |
| SMO | ite Management Organization | 现场管理组织 |
| | Investigational Site/Study site | 研究中心 |
| PI | Principal Investigator | 主要研究者 |
| | Investigator | 研究者 |
| CI | Co-investigator | 合作研究者 |
| COI | Coordinating+Investigator | 协调研究者 |
| SI | Sub-investigator | 助理研究者 |
| | Inspection | 视察、检查 |
| | Audit | 稽查 |
| | Auditor | 稽查员 |
| | audit certificate | 稽查证书 |
| | Monitor | 监查员 |
| | Monitoring | 监查 |
| | Monitoring reports | 监查报告 |
| CRC | Clinical Research Coordinator | 临床研究协调员 |
| | Contract/Agreement | 合同/协议 |
| CV | curriculum vitae | 简历 |
| CTP | Clinical Trial Protocol | 临床试验方案 |
| | Protocol Amendments | 修正案 |
| CTR | Clinical Trial Report | 临床试验报告 |
| CSR | Clinical Study Report | 临床研究报告 |
| IB | Investigator s Brochure | 研究者手册 |
| ICF | Informed Consent Form | 知情同意书 |
| CRF | Case Report Form | 病历报告表 |
| EDC | Electronic Data Capture | 电子数据采集 |
| IVRS | Interactive Voice Response System | 交互式语音应答系统 |
| IWRS | Interactive Web Response System | 交互式网络应答系统 |
| SIV | Site Initiation Visit | 中心启动访视 |
| ІМ/КОМ | Initial Meeting/kick-off meeting | 启动会 |
| OS | Overall Survival | 总生存期 |
| OA | Outcome Assessment | 结果评价 |
| PFS | Progression Free Survival | 无进展生存期 |
| QA | Quality Assurance | 质量保证 |
| QC | Quality Control | 质量控制 |
| SD | Source Data/document | 原始数据/文件 |
| SDV | Source Data Verification | 原始数据核查 |
| SOP | Standard Operating Procedure | 标准操作规程 |
| Ab | Abstact | 摘要 |
| | Study Objective | 研究目的 |
| | Statistical Methods | 统计学方法 |
| | Study Design | 研究设计 |
| | Exclusion Criteria | 排除标准 |
| | Inclusion Criteria | 入组标准 |
| | Study Endpoint | 研究终点 |
| | Study Procedure | 研究流程 |
| | Study Completion | 研究结束 |
| | Study Termination | 研究终止 |
| | Baseline | 基线 |
| | Blank Control | 空白对照 |
| | Randomized, Double-blind Study | 随机对照双盲试验 |
| | Single Blinding | 单盲 |
| | Double Blinding | 双盲 |
| | Blinding/Masking | 盲法/设盲 |
| | Unblinding | 揭盲/破盲 |
| | Active Control | 阳性对照、活性对照 |
| | Placebo | 安慰剂 |
| | Placebo Control | 安慰剂对照 |
| PV/PD | Protocol violation /Protocol deviation | 方案违背/方案偏离 |
| | Case Record/Medical History | 病历 |
| | Recruitment | 招募 |
| | Screening | 筛选 |
| | Randomization | 随机 |
| | Enrollment | 入组 |
| FPI/FSI | First Patient/Subject In | 首例受试者入组 |
| LPI/LSI | Last Patient/Subject In | 末例受试者入组 |
| LPO/LSO | Last Patient/Subject Out | 未例受试者出组 |
| | Cycle | 周期 |
| | Follow Up | 随访 |
| | Visit | 访视 |
| | Visit Window | 访视窗口期 |
| | Out of Visit Window | 超窗 |
| | Treatment Allocation | 治疗分配 |
| | Wash-out Period | 洗脱期 |
| | Withdrawal | 脱落 |
| | Compliance | 依从性 |
| | Demography | 人口统计学 |
| | Race | 种族 |
| | Weigh | 体重 |
| | Height | 身高 |
| | BMI | 身体质量指数 |
| DOB | Date of Birth | 出生日期 |
| ECG | Electrocardiogram | 心电图 |
| | Image | 影像学 |
| | Chest X-ray | 胸部X光 |
| | Patient File | 病人档案 |
| | Physical Exam | 体格检查 |
| VS | Vital Signs | 生命体征 |
| SBP | Systolic Blood Pressure | 收缩压 |
| DBP | Diastolic Blood Pressure | 舒张压 |
| | Pulse/Heart rate | 脉搏/心率 |
| TEMP | Temperature | 体温 |
| | Subject | 受试者 |
| SD | Source Data/Document/Documentation | 原始数据/文件 |
| | Subject Diary | 受试者日记 |
| | Subject Screening Log | 受试者筛选表 |
| | Subject Enrollment Log | 受试者入选表 |
| | Subject Identification Code | 受试者识别代码 |

 联系我们
联系我们 地址:郑州市康复前街3号(郑州火车站西广场对面)。
地址:郑州市康复前街3号(郑州火车站西广场对面)。 乘车路线:乘坐40、夜班Y17、夜班Y2、G126、G81、201、70、256、58、729、217、20、981、213、176至火车站西广场(郑州大学第五附属医院)站下车。
乘车路线:乘坐40、夜班Y17、夜班Y2、G126、G81、201、70、256、58、729、217、20、981、213、176至火车站西广场(郑州大学第五附属医院)站下车。 地铁路线:乘坐地铁1号线至郑州火车站从C口出站。
地铁路线:乘坐地铁1号线至郑州火车站从C口出站。 急救电话:0371-6690 2126 / 6690 2120 (24小时)
急救电话:0371-6690 2126 / 6690 2120 (24小时)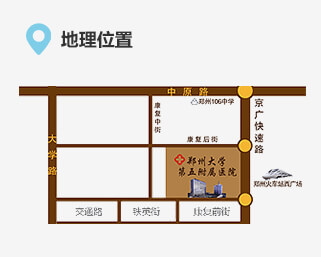 乘车路线:乘坐40、夜班Y17、夜班Y2、G126、G81、201、70、256、58、729、217、20、981、213、176至火车站西广场(郑州大学第五附属医院)站下车。地铁路线:乘坐地铁1号线至郑州火车站从C出口出站
乘车路线:乘坐40、夜班Y17、夜班Y2、G126、G81、201、70、256、58、729、217、20、981、213、176至火车站西广场(郑州大学第五附属医院)站下车。地铁路线:乘坐地铁1号线至郑州火车站从C出口出站







 李玉萍
李玉萍 杨楠
杨楠 芦秀琼
芦秀琼 田晓庚
田晓庚



 首页
首页




